An international, interdisciplinary team of scientists, led by the Luxembourg of Health (LIH), has been able to show that the cells of Glioblastoma – an extremely aggressive type of brain tumour – can adapt to their environment and transform their surface structure. The new insights could help optimise future treatments.
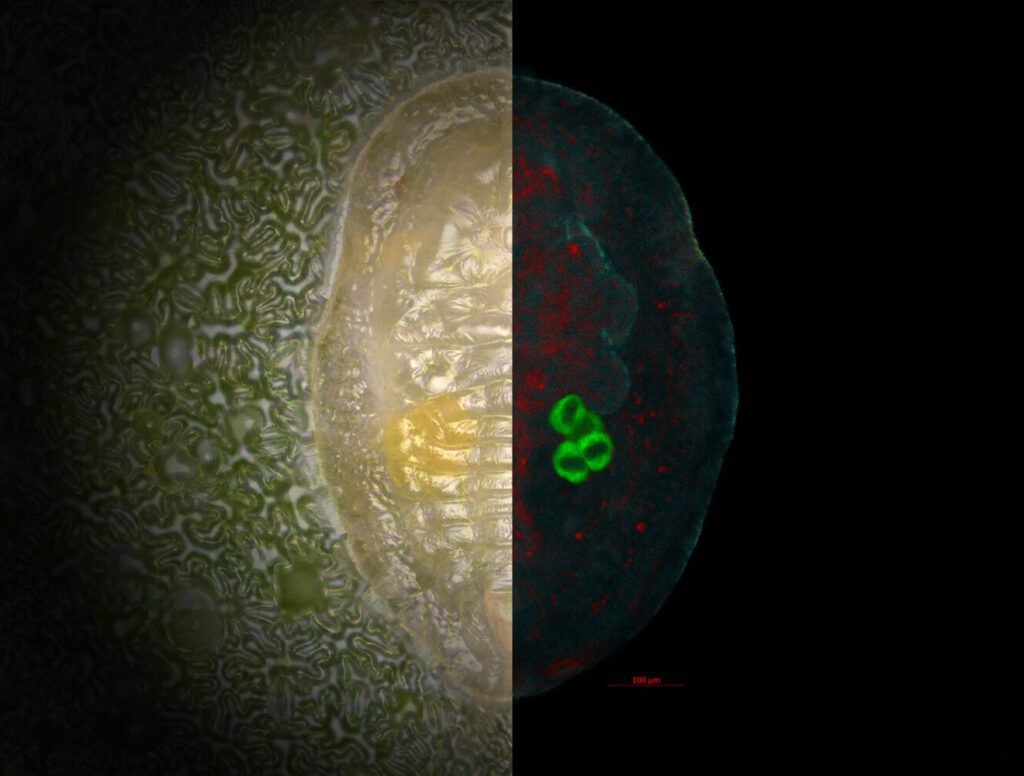
Glioblastomas are the most common malignant brain tumors. Glioblastomas have finger-like tentacles that infiltrate the brain, which makes them very difficult to remove completely. Because of their rapid growth, the prognosis for patients is usually dismal: With standard treatment, (surgery, radiation and chemotherapy) survival after diagnosis is only 12 – 15 months on average, with less than 5% of patients surviving longer than five years. Notable people who have died of Glioblastoma include US Senator John McCain and Ted Kennedy, son of John F. Kennedy.
Many patients hold out hope for novel therapeutic approaches, which utilise drug-bound antibodies directed against specific markers present on the surface of a subpopulation of immature glioblastoma cells. These antibody-drug conjugates bind to the surface, are then internalised and kill the cancer stem cells.
Implications for therapies involving targeting of cancer stem cells
Until now, scientists have assumed that the growth of solid tumours (tumours that do not contain any liquid or cysts) originates from cancer stem cells, characterised by specific surface markers, which develop in a fixed, hierarchical order. Such cancer stem cells play a key role in the tumour progression, and they produce specific types of more differentiated cancer cells whose fates are predetermined.
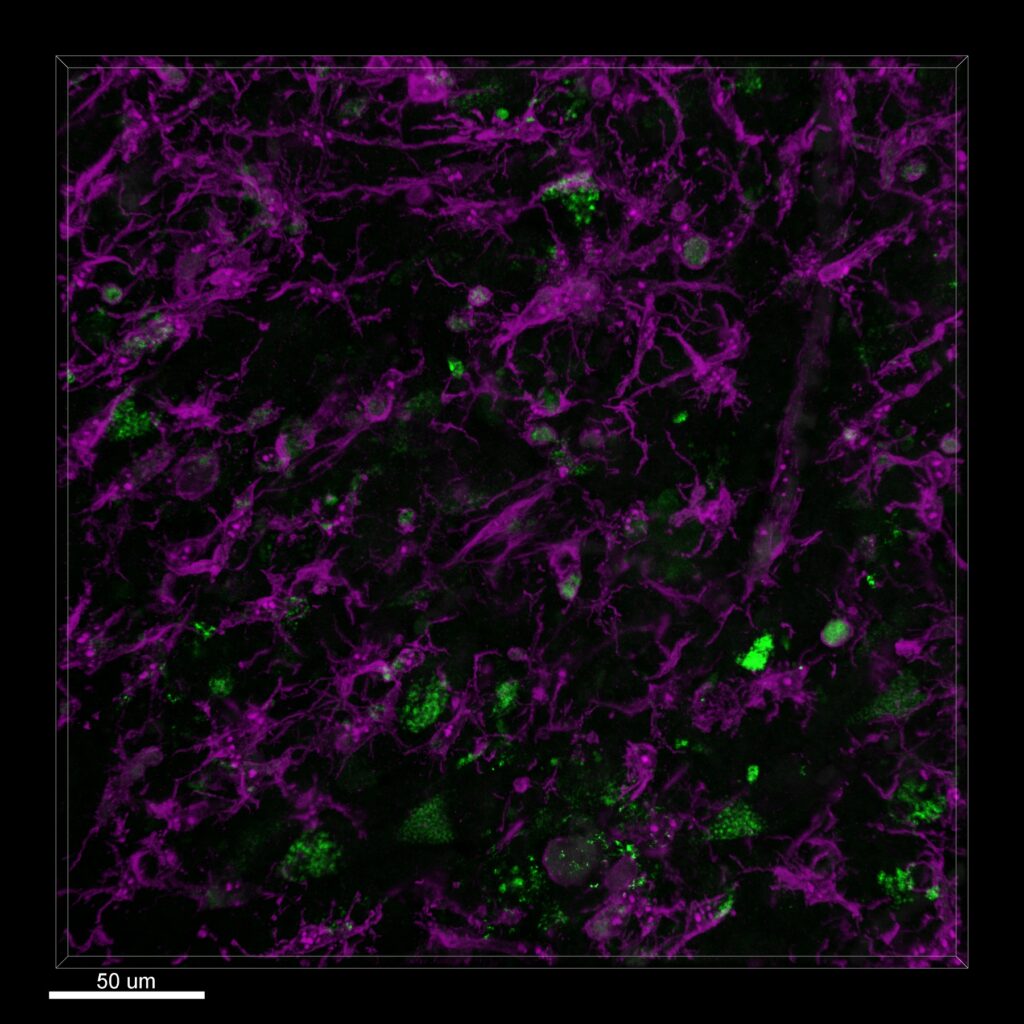
However, a joint interdisciplinary project led by the Luxembourg Institute of Health (LIH), researchers have shown that cancer cells of glioblastomas manifest developmental plasticity – the ability to change their physiological characteristics. The scientists also found that the phenotypic characteristics of glioblastoma cells are less constrained than believed.
Cancer stem cells, including their ‘offspring’, can adapt to environmental conditions and undergo reversible transformations into various cell types, thereby altering their surface structures. The results imply that novel therapeutic approaches, which target specific surface structures of cancer stem cells, will be of limited utility.
“We exposed cancer cells in the laboratory to certain stressors, such as drug treatment or oxygen deficiency,” explains Dr. Anna Golebiewska, Junior Principal Investigator at the NORLUX Neuro-Oncology Laboratory in LIH’s Department of Oncology and co-first author of the study.
“We were able to show that glioblastoma cells react flexibly to such stress factors and simply transform themselves at any time into cell types with a different set of surface markers.”
This plasticity allows the cells to adapt to their microenvironment and reach a favorable environment-specific heterogeneity that enables them to sustain and grow, and mostly likely also to escape therapeutic attacks.
Same phenomenon observed in breast and skin cancer
The team of scientists from Luxembourg, Norway and Germany, led by Prof. Simone P. Niclou at LIH, proposes that neoplastic cells of other tumor types may be also less constrained by defined hierarchical principles, but rather can adapt their characteristics to the prevailing environmental conditions.
“The same phenomenon has been observed in breast and skin cancer,” says Dr. Golebiewska. “This observation predicts that cancer therapies specifically directed against cancer stem cell markers may not be successful in patients.”
Using new insights to improve treatment
The new findings could help optimise future standard treatments. In laboratory experiments, the researchers were able to show that environmental factors, such as lack of oxygen in combination with signals from the tumor microenvironment can induce cancer cells to modify their characteristics.
This microenvironment, the immediate surrounding of the cancer, comprises cells and molecules that influence the growth of the tumor. “Once we understand exactly what causes the plasticity of tumor cells, we can devise combination therapies which target the signals underlying plasticity and thereby improve the therapeutic impact,” underlines Dr. Golebiewska.
Collaboration and funding
The study is a collaborative work between the NORLUX Neuro-Oncology Laboratory and other research units and platforms at LIH. The researchers from LIH also worked in close collaboration with their long-term national partners to whom they are tightly connected through transversal research programmes: the Luxembourg Centre for Systems Biomedicine at the University of Luxembourg and the Department of Neurosurgery of the Centre Hospitalier de Luxembourg. Moreover, the project was carried out with international partners from the Technische Universität Dresden, Germany, the University of Heidelberg, Germany, and the University of Bergen, Norway. This joint undertaking of different research and clinical players gives a truly interdisciplinary dimension to the study.
The study is a major part of the AFR PhD thesis of Dr Anne Dirkse, co-first author on the publication, who was supported by an AFR PhD grant and a training grant from the Fondation du Pélican de Mie et Pierre Hippert-Faber (Fondation de Luxembourg). Furthermore, the work was supported by funding from LIH, Sächsisches Staatsministerium für Wissenschaft und Kunst (SMWK), Deutsche Krebshilfe and Deutsche Forschungsgemeinschaft (DFG).
Publication
The research team published its findings (open access) in Nature Communications in April 2019. (Dirkse, Golebiewska et al. Nature Communications 2019, DOI: 10.1038/s41467-019-09853-z).